27+ pages how many moles of the excess reactant remain 810kb. Explanationoxygen was the most abundant element earths crust. Excess Reactant - The reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed. This chemistry video tutorial explains how to find the amount of excess reactant that is left over after the reaction is complete. Read also excess and understand more manual guide in how many moles of the excess reactant remain 2 P 5 F 2 2 PF 5 a.
Basically when a reactant is added more than the required amount to completely react out another compound the reactant is said to be in excess. What is limiting reactant.
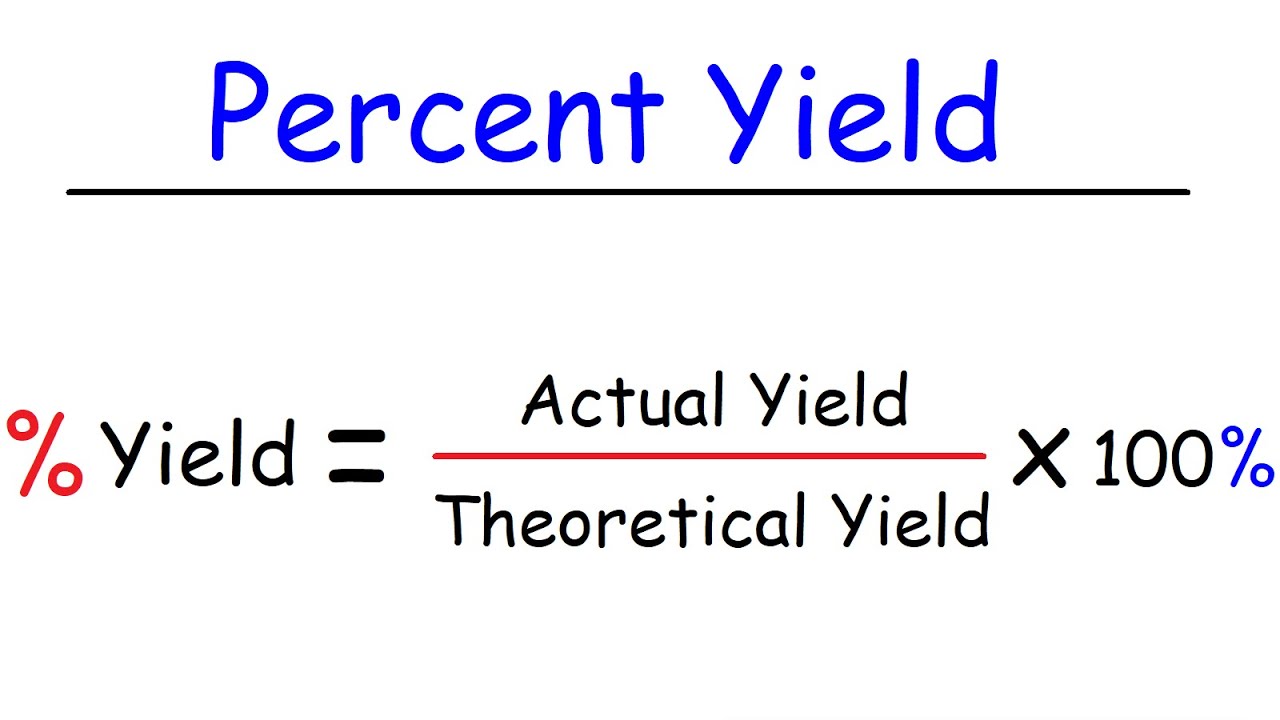
How To Find The Amount Of Excess Reactant That Is Left Over Chemistry
| Title: How To Find The Amount Of Excess Reactant That Is Left Over Chemistry |
| Format: PDF |
| Number of Pages: 309 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: January 2017 |
| File Size: 810kb |
| Read How To Find The Amount Of Excess Reactant That Is Left Over Chemistry |
 |
You need to start with th.

1 mole of ozone reacts with 1 mole of NO. 1B 2C 3B 4A 5A 6A 7C. What does in excess mean in chemistry. Ag 2 Bg 3 Cg a 0100 mol A b 0200 mol A c 0200 mol B d 0300 mol B. How many grams of the excess reactant remain. Which reactant is in excess.

Limiting Reactant Practice Problem Advanced
| Title: Limiting Reactant Practice Problem Advanced |
| Format: eBook |
| Number of Pages: 315 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: January 2021 |
| File Size: 3.4mb |
| Read Limiting Reactant Practice Problem Advanced |
 |

Calculating The How Much Excess Reactant Remains
| Title: Calculating The How Much Excess Reactant Remains |
| Format: eBook |
| Number of Pages: 167 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: March 2021 |
| File Size: 810kb |
| Read Calculating The How Much Excess Reactant Remains |
 |

The Bca Method In Stoichiometry Ppt Video Online Download
| Title: The Bca Method In Stoichiometry Ppt Video Online Download |
| Format: ePub Book |
| Number of Pages: 164 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: May 2021 |
| File Size: 1.9mb |
| Read The Bca Method In Stoichiometry Ppt Video Online Download |
 |

Chemical Equilibrium Notes And Ice Method Chemistry Notes Engineering Notes Teaching Chemistry
| Title: Chemical Equilibrium Notes And Ice Method Chemistry Notes Engineering Notes Teaching Chemistry |
| Format: ePub Book |
| Number of Pages: 174 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: June 2021 |
| File Size: 1.7mb |
| Read Chemical Equilibrium Notes And Ice Method Chemistry Notes Engineering Notes Teaching Chemistry |
 |
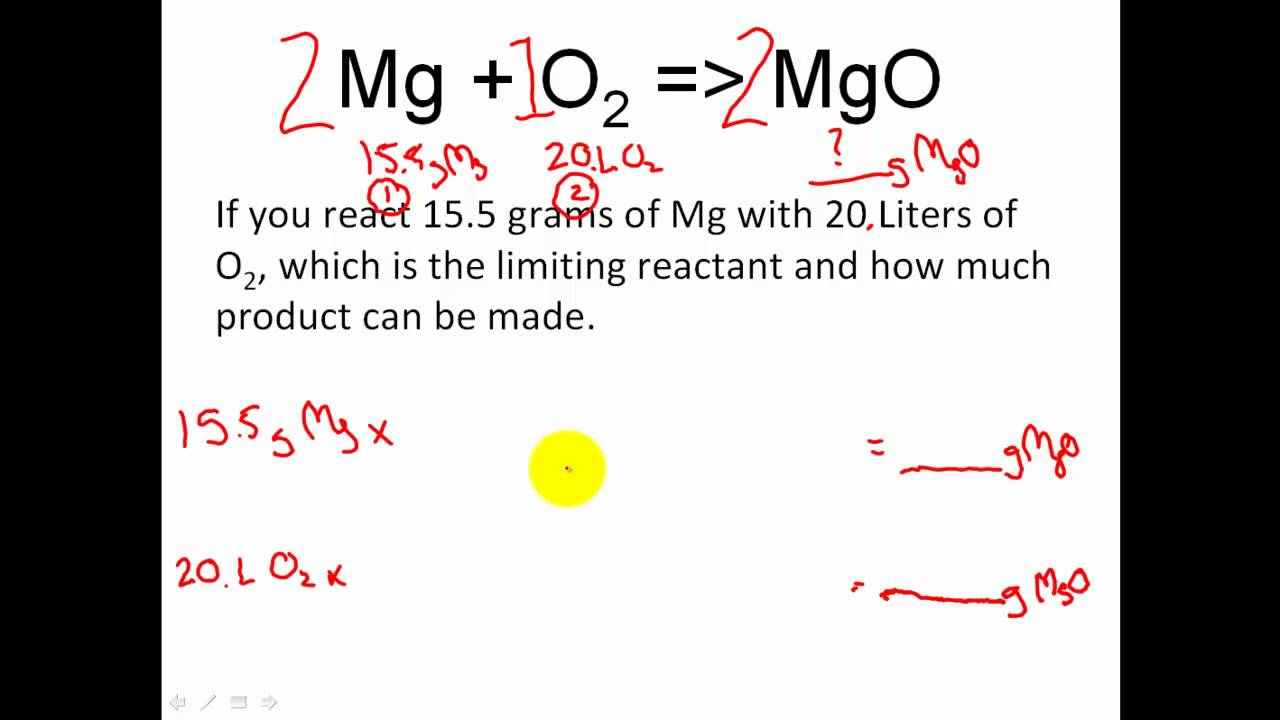
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles
| Title: Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles |
| Format: ePub Book |
| Number of Pages: 309 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: April 2021 |
| File Size: 2.8mb |
| Read Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles |
 |
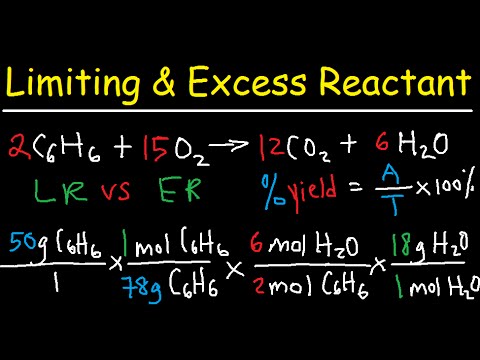
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry
| Title: Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry |
| Format: eBook |
| Number of Pages: 324 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: October 2020 |
| File Size: 1.35mb |
| Read Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry |
 |

How To Find How Much Excess Reactant Remains Examples Practice Problems Questions Summary
| Title: How To Find How Much Excess Reactant Remains Examples Practice Problems Questions Summary |
| Format: ePub Book |
| Number of Pages: 150 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: January 2020 |
| File Size: 1.8mb |
| Read How To Find How Much Excess Reactant Remains Examples Practice Problems Questions Summary |
 |

Limiting Reactant Example Problem 1 Edited Video Khan Academy
| Title: Limiting Reactant Example Problem 1 Edited Video Khan Academy |
| Format: eBook |
| Number of Pages: 214 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: February 2019 |
| File Size: 2.6mb |
| Read Limiting Reactant Example Problem 1 Edited Video Khan Academy |
 |

How To Find The Amount Of Excess Reactant That Is Left Over Chemistry
| Title: How To Find The Amount Of Excess Reactant That Is Left Over Chemistry |
| Format: eBook |
| Number of Pages: 338 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: May 2018 |
| File Size: 2.2mb |
| Read How To Find The Amount Of Excess Reactant That Is Left Over Chemistry |
 |

On Science Edu Chemkate
| Title: On Science Edu Chemkate |
| Format: eBook |
| Number of Pages: 336 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: July 2021 |
| File Size: 1.3mb |
| Read On Science Edu Chemkate |
 |

Reaction Stoichiometry Boundless Chemistry
| Title: Reaction Stoichiometry Boundless Chemistry |
| Format: eBook |
| Number of Pages: 296 pages How Many Moles Of The Excess Reactant Remain |
| Publication Date: January 2018 |
| File Size: 1.7mb |
| Read Reaction Stoichiometry Boundless Chemistry |
 |
2 molecules Construct a Before-Change-After Table for this reactant mixture. If you react with 10 grams of salicylic acid and 10 grams of acetic anhydride How many grams of the excess reactant remain when the reaction is complete. Ag 2 Bg 3 Cg a 0100 mol A b 0200 mol A c 0200 mol B d 0300 mol B.
Here is all you need to read about how many moles of the excess reactant remain Ag 2 Bg 3 Cg a 0100 mol A b 0200 mol A c 0200 mol B d 0300 mol B. When 368 g of benzene react with 400 grams of Chlorine which reactant will limit the amount of product produced. 1 mole of ozone reacts with 1 mole of NO. Chemical equilibrium notes and ice method chemistry notes engineering notes teaching chemistry stoichiometry limiting excess reactant theoretical percent yield chemistry limiting reactant example problem 1 edited video khan academy limiting reactant practice problem advanced reaction stoichiometry boundless chemistry the bca method in stoichiometry ppt video online download 4Al 3O2 2Al2O3 aPowdered Al 0048 mol is placed into a container containing 0030 mol O2.